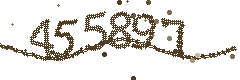
Question Bank: Homework Help for Any Subject
Welcome to Question Bank, the ultimate homework learning platform brought to you by the dedicated tutor team at UpStudy!
Here, all the questions come from real stude...
Load more
error msg
Homework Origins and How Question Bank Helps
Who Invented Homework?
Homework dates back to the late 19th century, often being credited to an Italian educator named Roberto Nevilis as its creator. Nevilis introduced homework-like assignments as an extra means of reinforcing education outside school hours; although homework-type assignments existed prior to Nevilis. Indeed, ancient civilizations such as Greeks and Romans utilized similar strategies of reinforcing learning outside classroom hours. While Nevilis formalized this practice into homework as we know it today; its meaning has significantly evolved with different cultural and educational influences shaping its current meaning over time.
Does Homework Help Students Learn?
Yes, homework can be a useful tool for reinforcing what children learn in the classroom, but its efficacy is dependent on numerous things. According to research, homework that is purposeful and balanced can help students acquire crucial skills such as time management, critical thinking, and problem solving. However, excessive or badly designed schoolwork can cause stress and exhaustion. Homework works best when it is designed to reinforce key concepts, foster independent thinking, and provide opportunities for personal reflection. So, while homework can be beneficial, the trick is determining the appropriate amount and manner to help pupils learn without overwhelming them.
How Can Question Bank Help You?
Question Bank by UpStudy is an invaluable tool designed to aid students in tackling homework across an array of subjects. As an extensive question bank that mirrors real student homework problems, Question Bank ensures the content remains practical and applicable for the task at hand. No matter your subject matter--be it math, science or history - Question Bank provides questions curated specifically to match assignments from real students; helping you practice key concepts while simultaneously preparing you for exams by offering expertly sourced questions that enable smart studying with deeper material grasp and greater insight!
Please type the verification code shown: